|
| Condition |
Imaging Findings |
Comments |
|
| Bacterial pneumonia |
Staphylococcus
(Fig C 1-1) |
Rapid development of extensive alveolar infiltrates, usually involving a whole lobe or even several lobes. Air bronchograms are infrequent because the acute inflammatory exudate fills the airways, leading to segmental collapse and a loss of volume. |
Most frequently occurs in children, especially during the first year of life. In adults, usually affects hospitalized patients with lowered resistance or as a complication of a viral respiratory infection. A characteristic finding in childhood disease is the development of pneumatoceles, thin-walled cystic spaces in the parenchyma that typically disappear spontaneously within several weeks. Pleural effusion (or empyema) often occurs. |
Streptococcus
(see Fig C 15-3) |
Indistinguishable from staphylococcal pneumonia. Homogeneous or patchy consolidation in a segmental distribution with a lower lobe predominance and often some loss of volume. |
Uncommon condition that usually follows viral infections such as measles, pertussis, and epidemic influenza. Unlike staphylococcal infection, streptococcal pneumonia rarely causes the development of pneumatoceles. Early and rapid accumulation of empyema fluid was a characteristic feature before the advent of antibiotics. |
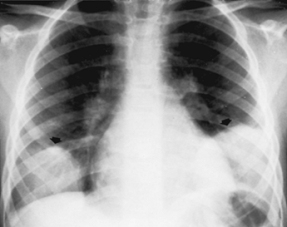
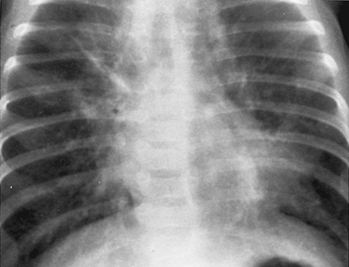
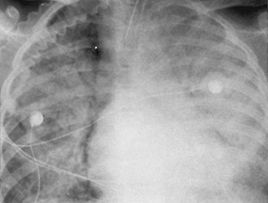
Pneumococcus
(Fig C 1-2) |
Homogeneous consolidation that almost invariably abuts against a visceral pleural surface and almost always contains an air bronchogram. |
Most commonly occurs in alcoholics and other compromised hosts. Cavitation and pleural reaction are rare. In children, may produce the so-called round or spherical pneumonia, in which a well-circumscribed spherical consolidation on both frontal and lateral views simulates a pulmonary or mediastinal mass (Fig C 1-3). |
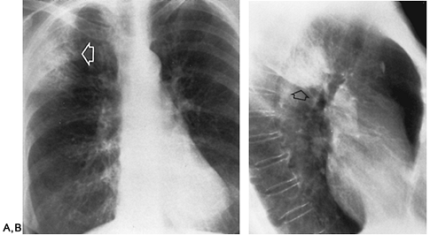
Klebsiella
(Fig C 1-4) |
Homogeneous parenchymal consolidation containing air bronchograms (simulates pneumococcal pneumonia). Primarily involves the right upper lobe. Typically induces a large inflammatory exudate, causing increased volume of the affected lobe and characteristic bulging of an adjacent interlobar fissure (see Fig C 15-1). |
Most commonly develops in alcoholics and in elderly patients with chronic pulmonary disease. Unlike acute pneumococcal pneumonia, Klebsiella pneumonia causes frequent and rapid cavitation, and there is a much greater incidence of pleural effusion and empyema. |
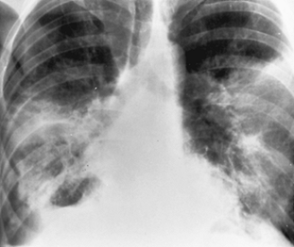
Other enteric gramnegative bacteria
(Fig C 1-5) |
Nonspecific, often inhomogeneous pattern of consolidation that most commonly affects the lower lobes. Cavitation is relatively common, and pleural effusion may occur. |
Escherichia coli, Serratia marcescens, Enterobacteriaceae, Proteus, Pseudomonas aeruginosa, Salmonella, and Brucella. Most commonly develop in debilitated or immunocompromised patients. |
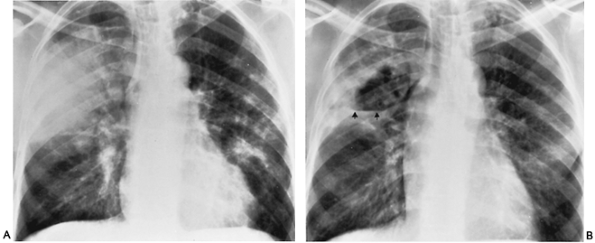
Haemophilus influenzae
(Fig C 1-6) |
Nonspecific patchy pulmonary infiltrate that is often bilateral. May be unilateral lobar or segmental consolidation, simulating pneumococcal disease. Typically extensive pleural involvement that often appears out of proportion to the associated parenchymal infiltrate. |
Serious infections primarily affect children under the age of 4 years and older patients who have undergone antibiotic therapy or who suffer from diseases that increase their general susceptibility to infection. This organism is the leading cause of epiglottitis (see Fig C 35-2), the second leading cause of childhood otitis media, and a common cause of childhood bacterial meningitis. |
Haemophilus pertussis
(whooping cough)
(Fig C 1-7) |
Various combinations of atelectasis, segmental pneumonia, and hilar lymph node enlargement. Coalescence of air-space consolidation contiguous to the heart produces a typical “shaggy heart†contour. |
Although often considered to have been largely eradicated by immunization, immunity is apparently not lifelong, and pertussis has become a not uncommon cause of bronchitis in adults. Acute infection most frequently affects nonimmunized children younger than 2 years of age.
|
Tularemia
(see Fig C 12-2) |
Patchy consolidations that may be bilateral, multilobar, or both. Ipsilateral hilar adenopathy and pleural effusion occur in approximately half the cases. |
Pneumonia represents hematogenous spread or inhalation of Francisella tularensis, which is usually transmitted to humans from infected animals (rodents, small mammals) or insect vectors. |
Yersinia pestis
(see Fig C 2-13 and Fig C 12-3) |
Patchy segmental infiltration or dense lobar consolidation simulating pneumococcal pneumonia. Typically, there is enlargement of hilar and paratracheal lymph nodes and, often, pleural effusion. |
The pneumonic type of plague causes severe pulmonary consolidation, necrosis, and hemorrhage and is usually fatal. This organism is still widespread among wild rodents. |
| Anthrax |
Patchy parenchymal infiltrates that are usually associated with pleural effusion and mediastinal widening (lymph node enlargement and hemorrhagic mediastinitis). |
Bacterial disease of cattle, sheep, and goats that primarily affects humans who inhale spores from infected animals or their products (eg, wool, hides). |
Legionnaires’ disease
(Fig C 1-8) |
Patchy or fluffy alveolar infiltrate that rapidly progresses to involve adjacent lobes and the contralateral side. |
Acute gram-negative bacterial pneumonia that occurs in local outbreaks or as sporadic cases and may cause a fulminant, often fatal, pneumonia. Small pleural effusions are common, whereas cavitation and hilar adenopathy are unusual. Most patients respond well to erythromycin, though the radiographic resolution often lags behind the clinical response. |
Bacteroides
(Fig C 1-9) |
Patchy or confluent consolidation that is generally confined to the lower lobes. Cavitation and empyema are common. |
Gram-negative anaerobic bacteria that are commonly found in the gastrointestinal and genital tracts. Pneumonia develops from aspiration of infected material or septic infarctions resulting from emboli arising in veins in the peritonsillar area or pelvis. |
|
| Fungal pneumonia |
Histoplasmosis
(Fig C 1-10) |
In the primary form, single or multiple areas of consolidation that are most often in the lower lung and associated with hilar lymph node enlargement. |
Striking hilar adenopathy, which may cause bronchial compression, may develop without radiographic evidence of parenchymal disease. Although the findings simulate primary tuberculosis, pleural effusion rarely occurs with histoplasmosis. |
Blastomycosis
(Fig C 1-11) |
Nonspecific patchy areas of air-space consolidation. |
Cavitation and miliary nodules infrequently occur. Blastomycosis may appear as a solitary pulmonary mass that, when associated with unilateral lymph node enlargement, may mimic a bronchogenic carcinoma. |
Coccidioidomycosis
(Fig C 1-12) |
Pulmonary involvement usually begins as a fleeting area of patchy pneumonia that is often accompanied by ipsilateral hilar adenopathy and, less frequently, by pleural effusion. |
Thin-walled cavities without surrounding reaction are suggestive of this organism (see Fig C 9-5). |
Cryptococcosis (torulosis)
(Fig C 1-13) |
Segmental or lobar consolidation that most commonly occurs in the lower lobes. |
More commonly produces a single, fairly well-circumscribed mass that is usually in the periphery of the lung and is often pleural based. Cavitation is relatively uncommon compared with its frequency in the other mycoses. |
Actinomycosis nocardiosis
(Fig C 1-14 and Fig C 1-15) |
Nonsegmental air-space consolidation (may resemble pneumonia or a tumor mass). Cavitation and empyema are common if not appropriately treated. |
Extension of the infection into the pleura produces an empyema, which classically leads to osteomyelitis of the ribs and the formation of a sinus tract.
|
| Candidiasis |
Patchy, segmental, homogeneous air-space consolidation. |
Reflects hematogenous dissemination. Cavitation and hilar adenopathy may occur. |
Aspergillosis
(see Fig C 20-1) |
Single or multiple areas of consolidation with poorly defined margins. |
Almost always a secondary infection in which the fungus colonizes a damaged bronchial tree, pulmonary cyst, or cavity of a patient with underlying lung disease. The radiographic hallmark is a pulmonary mycetoma, a solid homogeneous rounded mass separated from the wall of the cavity by a crescent- shaped air space. |
Mucormycosis
(see Fig C 9-7) |
Progressive severe pneumonia that is widespread and confluent and often cavitates. |
Occurs in patients with diabetes or an underlying malignancy (leukemia, lymphoma). Usually originates in the nose and paranasal sinuses, where the infection may destroy the walls and create an appearance that simulates a malignant neoplasm. |
Sporotrichosis
(see Fig C 9-6) |
Various nonspecific patterns (fibronodular infiltrates, cavitary nodular masses, chronic pneumonia). Hilar lymph node enlargement is common and may cause bronchial obstruction. Spread through the pleura into the chest wall may produce a sinus tract. |
Chronic infection that is usually limited to the skin and the draining lymphatics. In rare instances, disseminated disease can involve the lungs and the skeletal system (extensive destructive arthritis with large-joint effusions). |
|
Mycoplasma/viral infection
(Fig C 1-16 and Fig C 1-17) |
Patchy air-space consolidation that is usually segmental and predominantly involves the lower lobes. Bilateral and multilobar involvement is common. |
Initially, acute interstitial inflammation appears as a fine or coarse reticular pattern. Most infections are mild, though the radiographic signs are more extensive than might be expected from the physical examination. |
|
| Mononucleosis |
Nonspecific patchy air-space consolidation. |
Generalized lymphadenopathy and splenomegaly are characteristic findings. Hilar lymph node enlargement, usually bilateral, can be demonstrated in approximately 15% of cases (see Fig C 11-1). Pneumonia is a rare complication. |
|
| Varicella |
Extensive bilateral fluffy nodular infiltrate that tends to coalesce near the hilum and lung bases. |
Healed varicella pneumonia classically appears as tiny miliary calcifications (see Fig C 17-5), scattered widely throughout both lungs, which develop several years after the acute infection. |
|
| Cytomegalovirus |
In adults, rapid development of diffuse bilateral alveolar infiltrates that are most common in the outer third of the lungs. |
Primarily involves patients with underlying reticuloendothelial disease or immunologic deficiencies, or those receiving immunosuppressive therapy (especially after renal transplantation). May be radiographically indistinguishable from Pneumocystis carinii pneumonia.
|
|
Rickettsial infection
(Fig C 1-18) |
Dense, homogeneous, segmental, or lobar consolidation simulating pneumococcal disease. Predominantly affects the lower lobes and may be bilateral. |
Pneumonia develops in approximately half the patients with Q fever. Pleural effusion occurs in about one-third of the cases, whereas hilar involvement and small focal lesions are rare. |
| Parasitic pneumonia |
Pneumocystis carinii
(Fig C 1-19; see Fig C 2-14 and Fig C 4-19) |
Initially, a hazy, perihilar granular infiltrate that spreads to the periphery and appears predominantly interstitial. In later stages, patchy areas of airspace consolidation with air bronchograms. Massive consolidation with virtually airless lungs may be a terminal appearance. |
Common organism in immunosuppressed patients (especially those with AIDS and those treated for lymphoproliferative diseases or with renal transplants). Hilar adenopathy and significant pleural effusions are rare and should suggest an alternative diagnosis. Because the organism cannot be cultured and the disease it causes is usually fatal if untreated, an open-lung biopsy is often necessary if a sputum examination reveals no organisms in a patient suspected of having this disease. |
| Amebiasis |
Air-space consolidation in the right lower lobe that may be obscured by an extensive pleural effusion. |
Usually arises from direct extension of hepatic infection through the right hemidiaphragm (occasionally may be of hematogenous origin). |
| Toxoplasmosis |
Combined interstitial and alveolar disease, often with hilar lymph node enlargement. |
Especially virulent organism in immunocompromised patients. Central nervous system involvement is common and may lead to a brain abscess. |
Ascariasis
(see Fig C 18-4) |
Patchy or extensive areas of consolidation that are often bilateral. |
Reflects an allergic response caused by larvae migrating through the lungs. |
Cutaneous larva migrans (creeping eruption)
(see Fig C 18-7) |
Transient, migratory pulmonary infiltrates associated with lung and blood eosinophilia. |
Pulmonary involvement develops in approximately half the patients about 1 week after the skin eruption caused by penetration and migration of the larvae of the dog and cat hookworm (Ancylostoma braziliense). |
Strongyloidiasis
(see Fig C 18-5) |
Ill-defined patchy areas of air-space consolidation or fine miliary nodules. |
Pulmonary manifestations occur during the stage of larval migration (in most patients, the chest radiograph remains normal). |
Paragonimiasis
(see Fig C 7-3 and Fig C 9-9) |
Patchy air-space consolidation that primarily involves the bases of the lungs. Characteristic finding is the “ring shadow,†composed of a thin-walled cyst with a prominent crescentshaped opacity along one side of its border. |
Chronic infection of the lung caused by a trematode that is acquired by eating raw, or poorly cooked, infected crabs or crayfish. Although many patients with a heavy infestation are asymptomatic, others present with cough, pain, hemoptysis, and brownish sputum. |
|
| Tuberculosis |
Primary
(Fig C 1-20) |
In primary disease, a lobar or segmental airspace consolidation that is usually homogeneous, dense, and well defined. Associated enlargement of the hilar or mediastinal lymph nodes is very common (see Fig C 10-1 and Fig C 10-2). Pleural effusion often occurs, especially in adults (see Fig C 33-1). |
Primary tuberculosis may affect any lobe. The diagnosis cannot be excluded because the infection is not in the upper lobe. Although traditionally considered a disease of children and young adults, with the dramatic decrease in the prevalence of tuberculosis (especially in children and young adults), primary pulmonary disease can develop at any age.
|
| Secondary (reactivation) |
Initially a nonspecific hazy, poorly marginated alveolar infiltrate that most commonly affects the upper lobes, especially the apical and posterior segments. Cavitation is common (see Fig C 9-3) and may result in bronchogenic spread characterized by multiple patchy infiltrates. |
Bilateral (though often asymmetric) upper lobe disease is common and is almost diagnostic of reactivation tuberculosis. Because an apical lesion may be obscured by overlying clavicle or ribs, an apical lordotic view is often of value. Pleural effusion and lymph node enlargement are rare in secondary disease. |
|
Atypical mycobacteria
(see Fig C 9-4) |
Often radiographically indistinguishable from primary tuberculosis, though pleural effusion and hilar adenopathy are much less common. |
Often produces thin-walled cavities with minimal surrounding parenchymal disease. Patients with an atypical mycobacterial infection have a negative tuberculin test and do not respond to antituberculous therapy. |
|
Postobstructive pneumonitis
(Fig C 1-21) |
Homogeneous increase in density corresponding exactly to a lobe or one or more segments, usually with a substantial loss of volume. |
With slowly progressive, obstructive endobronchial processes such as bronchogenic carcinoma and bronchial adenoma, infection is frequent so that there may be only slight or moderate loss of volume. Pneumonitis, bronchiectasis, and abscesses that develop behind the obstruction are usually sufficient to counteract, at least partly, collapse induced by air absorption. The characteristic radiographic picture of “obstructive pneumonitis†should immediately suggest the presence of an obstructing endobronchial lesion. Nonneoplastic causes include mucoid impaction (hypersensitivity aspergillosis), aspirated foreign bodies, and the tracheobronchial form of amyloidosis. |
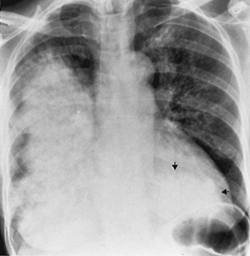
Pulmonary infarct
(Fig C 1-22) |
Area of consolidation that most commonly involves the lower lobes and is often associated with pleural effusion and elevation of the ipsilateral hemidiaphragm. A highly characteristic, though uncommon, appearance is a pleuralbased, wedge-shaped density that has a rounded apex (Hampton hump) and often occurs in the costophrenic sulcus. In many instances, an infarction produces a nonspecific parenchymal density that simulates an acute pneumonia. |
Although it is often said that infarction invariably extends to a visceral pleural surface, this is of little diagnostic value, as most pneumonias have a similar appearance. The pattern of resolution of the consolidation is of value in distinguishing among acute inflammatory processes, pulmonary hemorrhage, edema, and frank necrosis. Pulmonary infarctions tend to shrink gradually while retaining the same general configuration seen on initial views (resorption of the perimeter of the infarct with preservation of the pleural base). In contrast, the resolution of pneumonia tends to be patchy and is characterized by a fading of the radiographic density throughout the entire involved area. Parenchymal hemorrhage and edema generally clear within 4 to 7 days; the resolution of necrotic lung tissue usually requires 3 weeks or more. |
|
Pulmonary contusion
(see Fig C 6-14 and Fig C 31-2) |
Varies from irregular patchy areas of air-space consolidation to an extensive homogeneous density involving almost an entire lung. |
Most common pulmonary complication of blunt chest trauma in which there is exudation of edema and blood into both the air spaces and the interstitium of the lung. In the absence of an appropriate clinical history of trauma or evidence of rib fractures, pulmonary contusion may be indistinguishable from pneumonia. Resolution typically occurs rapidly, with complete clearing within 2 weeks.
|
|
Lipoid pneumonia
(Fig C 1-23) |
Granular pattern of small, scattered alveolar densities that predominantly occur in the perihilar and lower lobe areas. |
Caused by the aspiration of various vegetable, animal, or mineral oils into the lungs. As the oil is taken from the alveolar spaces by macrophages that pass into the interstitial space, a fine reticular pattern is produced. Infrequently appears as a granulomatous- lipoid mass that may be huge and may simulate bronchogenic carcinoma (see Fig C 6-15). |
|
| Lung torsion |
Opacification of the affected lung develops if the torsion is not relieved and the vascular supply is compromised. |
Rare complication of trauma that occurs almost invariably in children, presumably because of the easy compressibility of their thoracic cage. Torsion occurs through 180°, so that the base of the lung comes to lie at the apex of the hemithorax and the apex at the base. The pulmonary opacification is due to exudation of blood into the air spaces and interstitial tissues. |
|
Localized pulmonary edema
(Fig C 1-24) |
Nonsymmetric, atypical alveolar consolidation. |
Most commonly occurs in patients with pre-existing lung disease such as chronic emphysema. Unilateral pulmonary edema is most frequently related to dependency. |
|
Bronchioloalveolar (alveolar cell) carcinoma
(Fig C 1-25) |
In the less common diffuse form, a pattern varying from poorly defined nodules scattered throughout both lungs to irregular pulmonary infiltrates, often with air bronchograms. |
More frequently appears as a well-circumscribed, peripheral solitary nodule that often contains an air bronchogram (see Fig C 6-13) (never associated with solitary nodule caused by bronchogenic carcinoma or a granuloma). Although the margins of the tumor are usually well circumscribed, the mass may be poorly defined and simulate an area of focal pneumonia. |
|
| Lymphoma |
Patchy areas of parenchymal infiltrate that may coalesce to form a large homogeneous nonsegmental mass. Cavitation and pleural effusion may occur. |
Pleuropulmonary involvement usually occurs by direct extension from mediastinal nodes along the lymphatics of the bronchovascular sheaths. At times, it may be difficult to distinguish a superimposed infection after radiation therapy or chemotherapy from the continued spread of lymphomatous tissue. However, any alveolar lung infiltrate in a patient with known lymphoma is more likely to represent an infectious than a lymphomatous process. Primary pulmonary lymphoma is rare and presents as a homogeneous mass that rarely obstructs the bronchial tree and thus almost invariably contains an air bronchogram. When most or all of a segment or lobe is involved, the appearance may simulate acute pneumonia. |
|
| Pseudolymphoma |
Segmental consolidation extending outward from a hilum and containing an air bronchogram. |
Rare benign condition that histologically closely resembles malignant lymphoma. Although apparently segmental, in most cases the consolidation stops short of the visceral pleura at the periphery of the lung.
|
|
Löffler’s syndrome (idiopathic eosinophilic pneumonia)
(see Fig C 18-1) |
Transient, rapidly changing, nonsegmental areas of parenchymal consolidation associated with blood eosinophilia. The infiltrates are often located in the periphery of the lung, running more or less parallel to the lateral chest wall and simulating a pleural process. |
A similar appearance can develop secondary to parasites (filariasis, ascariasis, cutaneous larva migrans), drug therapy (nitrofurantoin), and fungal infections (hypersensitivity bronchopulmonary aspergillosis). When caused by an identifiable extrinsic agent, the disease usually is acute and responds promptly to the removal of the offending organism or drug. When no obvious cause is detectable, the pulmonary consolidation and eosinophilia tend to be more prolonged and persistent, though there is usually a dramatic response to steroids. |
|
Radiation pneumonitis
(Fig C 1-26) |
Patchy areas of irregular consolidation that are localized to the radiation port and are often associated with a considerable loss of volume. |
Acute radiation pneumonitis is rarely detectable less than 1 month after the end of treatment and must be differentiated from bacterial pneumonia. The late or chronic stage of radiation damage is characterized by extensive fibrosis and loss of volume that may be difficult to distinguish from the lymphangitic spread of a malignant tumor. |
|
Sarcoidosis
(see Fig C 2-17) |
Ill-defined densities that may be discrete or may coalesce into large areas of segmental consolidation. This pattern resembles an acute inflammatory process and may contain an air bronchogram. |
Infrequent manifestation. More characteristic radiographic changes are a diffuse reticulonodular pattern and typical bilateral enlargement of hilar and paratracheal lymph nodes (see Fig C 11-6 and Fig C 12-8). |
|
Progressive massive fibrosis (pneumoconiosis)
(see Fig C 7-9 and Fig C 7-10) |
Nonsegmental conglomerate masses that are usually bilateral and relatively symmetric and almost always restricted to the upper half of the lungs. They commonly develop in the mid-zone or periphery of the lung and tend to migrate later toward the hilum, leaving overinflated and emphysematous lung tissue between the consolidation and the pleural surface. |
Caused by the confluence of numerous individual nodules in patients with advanced silicosis or coalminer’s pneumoconiosis. The conglomerate fibrotic lesions may cavitate as a result of central ischemic necrosis or tuberculous caseation. |
|
| Asbestosis/talcosis |
In patients with extensive interstitial fibrosis, large conglomerate opacities may develop that are well or ill defined, are often multiple and nonsegmental, and predominantly involve the lower lung (in contrast to the upper lobe predominance of the conglomerate opacities in silicosis). |
Pleural plaque formation, which may be massive and bizarre in shape, is characteristic of both conditions (see Fig C 32-4 and Fig C 32-5). In asbestosis, there is often thin, curvilinear calcification of the diaphragmatic pleura, obscuration of the heart border (shaggy heart sign), and a high incidence of associated malignancy (bronchogenic carcinoma, mesothelioma). |
|
| Systemic lupus erythematosus |
Nonspecific patchy infiltrate that is more commonly situated peripherally in the lung bases. |
Often associated with bilateral pleural effusions and cardiac enlargement due to pericardial effusion (see Fig C 33-4). |



















Hi to all, it’s genuinely a nice for me to visit this web site, it consists of important Information.
Thanks
Good info. Lucky me I discovered your website by accident (stumbleupon).
I have bookmarked it for later!
Thanks